See my earlier posts on IRA worth negotiation on drug choice (Half 1) and producer information submission (Half 2).
Right now we’ll discuss concerning the negotiation course of and the way CMS will set the utmost truthful worth (MFP)
How will CMS worth throughout dosages?
“CMS will base the only worth on the price of the chosen
drug per 30-day equal provide (reasonably than per unit—similar to pill,
capsule, injection—or per quantity or weight-based metric), weighted throughout
dosage varieties and strengths.”
Is there a most worth or “ceiling” for the utmost
truthful worth (MFP) that CMS will supply?
The utmost MFP quantity will probably be no increased than:
- An quantity equal to the sum of the plan-specific
enrollment weighted quantities - The decrease of: the typical non-FAMP in 2021
elevated by inflation (CPI-U) or the typical non-FAMP worth in February 2025
CMS will combination the 60 quantities decided for every NDC-11 for the chosen drug to calculate a single quantity – individually for every methodology – throughout dosage varieties, strengths, and bundle sizes of the chosen drug. These quantities can then be straight in contrast, and the ceiling for the only MFP of the chosen drug (together with all dosage varieties and strengths) would be the decrease quantity.
Pattern packages, NDCs from secondary producers, NDCs
with no amount allotted or NDCs with gross coated prescription drug prices of
$0 is not going to be included within the MFP calculation.
Can some claims be excluded from the MFP refund?
As soon as the MFP worth is decided, there are some circumstances the place
a producer wouldn’t need to pay the MFP refund. These embody:
“…[justification] codes for the drug being prospectively bought at or under the MFP, the producer and allotting entity having a individually negotiated refund quantity distinct from the Normal Default Refund Quantity, and the declare being excluded from MFP refunds underneath part 1193(d)(1) of the Act”
CMS has to justify the MFP to producers. How will it do that?
The CMS justification will observe a 4-step course of:
- Identification of therapeutic different(s), if any, for the chosen drug. This contains FDA-approved medicine for the related indication and off-label use if included in nationally acknowledged, evidence-based pointers and in a CMS-recognized compendia. CMS will start by figuring out therapeutic options inside the identical pharmacologic class as the chosen drug primarily based on properties similar to chemical class, therapeutic class, or mechanism of motion, after which additionally contemplate therapeutic options in several pharmacologic lessons primarily based on CMS’ overview of related information (see query under).
- Measure the value of the therapeutic options. For Half D medicine, that is whole gross coated drug value (TGCDC) web of DIR and CGDP funds and/or the Common Gross sales Worth (ASP) for Half B medicine (or prior yr MFP if relevant)
- Decide if drug has distinctive profit. Consider whether or not the chosen drug—relative to therapeutic options—addresses an unmet want, has a helpful influence on IRA particular populations, and the extent to which the chosen drug represents a therapeutic advance in comparison with therapeutic different(s)
- Additional adjustment of preliminary worth. These changes will probably be primarily based on producer submitted information together with: (1) R&D prices and R&D prices recouped, (2) present unit prices of manufacturing and distribution; (3) prior Federal monetary assist for novel therapeutic discovery and growth; (4) pending and accepted patent purposes or exclusivities; and (5) market information and income and gross sales quantity information for the drug within the US., and (6) non-compulsory producer submitted information.
What information does CMS use to find out therapeutic options?
“…CMS will use information submitted by the Main Producer and the general public, FDA-approved indications, drug classification methods generally used within the public and business sector for formulary growth, CMS-recognized Half D compendia, broadly accepted medical pointers, the CMS led literature overview, drug or drug class opinions, and peer-reviewed research.”
How may CMS set the preliminary worth supply?
The first approach CMS will set it’s preliminary worth supply for
2027 is predicated on the web worth of therapeutic options.
Nonetheless…
If the chosen drug has no therapeutic different, if the costs of all therapeutic options recognized are above the statutory ceiling for the MFP…or if there’s a single therapeutic different for the chosen drug and its worth is above the statutory ceiling for the MFP, then CMS will decide the start line for the preliminary supply primarily based on the FSS or…“Huge 4 worth”…whichever is decrease. If the FSS and Huge 4 costs are above the statutory ceiling, then CMS will use the statutory ceiling as the start line for the preliminary supply.
Why did CMS select to set it’s preliminary worth primarily based on the
worth of therapeutic options?
Be aware that CMS did contemplate quite a lot of choices for setting
the preliminary worth supply together with web costs, unit value of manufacturing/distribution,
home references worth to the Federal Provide Schedule (FSS) worth, a “truthful
revenue” worth primarily based on whether or not R&D prices have been recouped and margin on
unit value of manufacturing and distribution, however settled on the web worth of
therapeutic options.
Nonetheless, it argues that the online worth of therapeutic options—regardless of
limitations—is a most popular possibility:
“In taking this method, CMS acknowledges that the therapeutic different(s) for a particular drug might not be priced to mirror its medical profit, nevertheless, utilizing Web Half D Plan Cost and Beneficiary Legal responsibility, ASPs, or MFPs of therapeutic options allows CMS to start out creating the preliminary supply inside the context of the fee and medical advantage of a number of medicine that deal with the identical illness or situation. Through the use of the value(s) of the chosen drug’s therapeutic different(s), CMS will have the ability to focus the preliminary supply on part 1194(e)(2) elements by adjusting this place to begin relative as to if the chosen drug presents extra, much less, or related profit in comparison with its therapeutic different(s).”
What elements will influence CMS’s resolution to regulate its
preliminary supply?
Some concerns embody:
- Scientific profit conferred by the chosen drug
in comparison with its therapeutic different(s), - Influence on patient-reported outcomes and affected person
expertise - Influence on caregivers
- Utilization patterns of the chosen drug versus its
therapeutic different(s) - Suggestions from consultations with clinicians,
sufferers or affected person organizations, tutorial specialists, and/or the FDA - Influence on CMS particular populations (people
with disabilities, the aged, people who’re terminally ailing, youngsters,
and different Medicare beneficiaries) - Whether or not or not the therapy meets an unmet
medical want
Key related info that will probably be thought-about embody: “…peer-reviewed
analysis, skilled stories or whitepapers, clinician experience, real-world
proof, and affected person expertise.” Key
outcomes of curiosity to be thought-about embody quite a lot of outcomes, together with
patient-centered outcomes, and affected person expertise.
Though CMS notes that it’ll not use cost-effectiveness
evaluation primarily based on QALYs, it has not dominated on whether or not it will possibly use different
approaches similar to equal worth of life years gained (evLYG), well being years in
whole (HYT) or generalized and risk-adjusted QALYs (GRA-QALYs).
These elements will influence the value by a qualitative resolution
course of.
Will caregiver expertise influence CMS choices?
Sure. The
steering says that “CMS may contemplate the caregiver perspective to the
extent that it displays straight upon the expertise or related outcomes of
the affected person taking the chosen drug.”
Does CMS contemplate value when evaluating if a therapy is
a therapeutic advance?
Sure.
“CMS will decide the extent to which a particular drug represents a therapeutic advance as in comparison with its therapeutic different(s) by inspecting enhancements in outcomes in comparison with its therapeutic different(s) (e.g., chosen drug is healing versus a therapeutic different that delays development) and can contemplate the prices of such therapeutic different(s). CMS could contemplate a particular drug to symbolize a therapeutic advance if proof signifies that the chosen drug represents a considerable enchancment in outcomes in comparison with the chosen drug’s therapeutic different(s) for a sign(s).”
How will the negotiation course of work?
That is summarized within the graphic under.
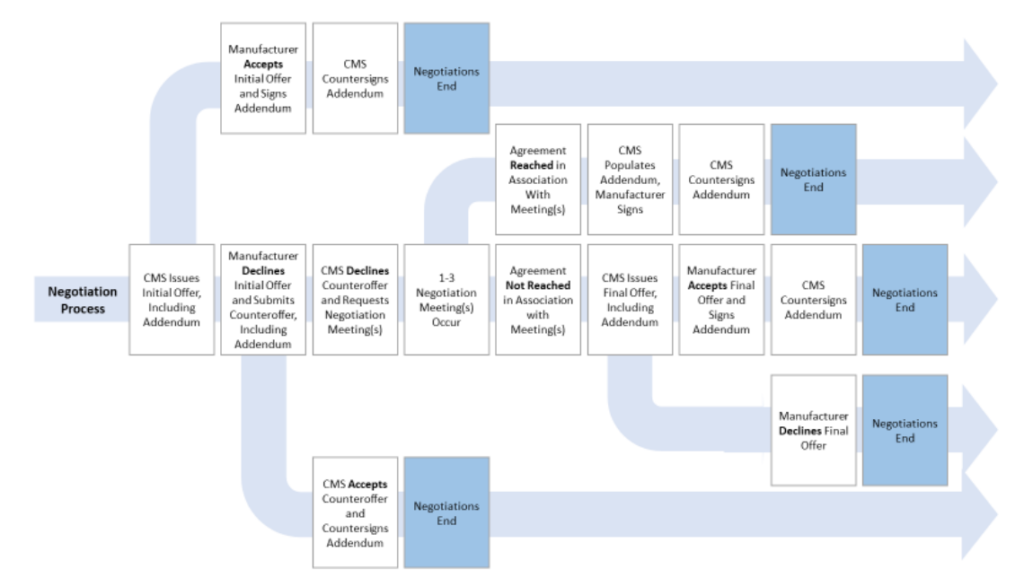
Extra element may be discovered within the CMS steering doc right here.
